Alzheimer’s Disease Model in Mice

Alzheimer’s Disease Model in Mice
Alzheimer’s disease (AD) is a progressive, neurodegenerative disease that impacts memory and behavior. Individuals with AD typically lose memories, show changes in personality, and may develop abnormal sleep patterns and seizures [1, 2]. One in 10 people age 65 and older has AD. In 2015, the worldwide prevalence of AD was 46.8 million people and by 2050 the prevalence will be close to 130 million [3]. Although a minority of AD are inherited or “Familial AD” (FAD), most cases of Alzheimer’s disease are sporadic, and the exact cause of AD is still unknown.
Induction:
Using the mutated genes found in FAD, several transgenic mouse models have been generated that mimic AD. Depending on the mutation and the number of mutated genes, mice will develop memory impairments around 3-6 months of age. We currently purchase established models from rodent vendors and measure the customer’s phenotype of interest at the appropriate time. For example, the Tg2576 model carries the human APP transgene with one single mutation that has been found in AD. These mice will develop neuropathology (extracellular plaques and gliosis between the age of 10-16 months), synaptic deficits (obtained with electrophysiology) and have impaired learning and memory (emerging between 3 and 6 months of age). Although rodents do not develop dementia the way humans do, these transgenic mice show similarities with human AD cases that allow them to serve as representative models to study the disease [4].
If working with the Tg2576 mice, 12 female transgenics and 12 female wild-type littermates (normal mice) will be ordered from Taconic and arrive at our facility at the age of 10 months. Females tend to have a better phenotype in this model, but males can also be ordered. After arrival, animals will be habituated to the facility for at least four weeks. Besides the daily health checks, no special care is needed for these mice.
A summary of a typical experimental design is laid out below:
Disease Parameters & clinical assessment:
During habituation to the facility and the behavioral assays, animals will be weighed weekly. Animals may be tested for several behavioral assays in the following order, starting at the age of 11 months:
- Open field assay (OFA): to test for locomotor performance and anxiety
- Elevated plus maze (EPM): to test for anxiety
- Morris water maze (MWM) or Barnes maze: depending on the physique of the animals, cognition may be tested using the Barnes maze or the MWM. The MWM is known to be more robust concerning cognition testing, however it may be too stressful for the animals. As an alternative, we can use the Barnes maze to test for cognition, however, external motivation for the animal to perform the task may be more variable
- Gait analysis: analysis for movement abnormalities
In comparison to the wild-type controls, transgenic AD mice will develop cognitive impairments over time and are therefore expected to show impaired performance on the Morris water maze and/or Barnes maze.
After being tested for locomotor performance and cognition, which will last approximately 1-2 months, animals will be euthanized and tissue may be taken at the age of 12-13 months.
Histopathological Assessment:
- Synaptic loss (≥5 months)
- Microglial activation (≥10 months)
- Astrogliosis (≥10 months)
- Amyloid-b expression (≥11 months)
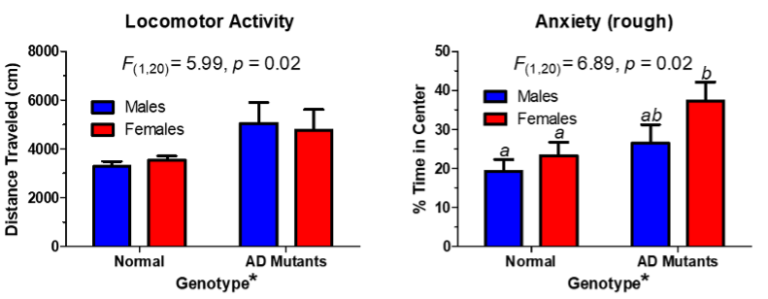
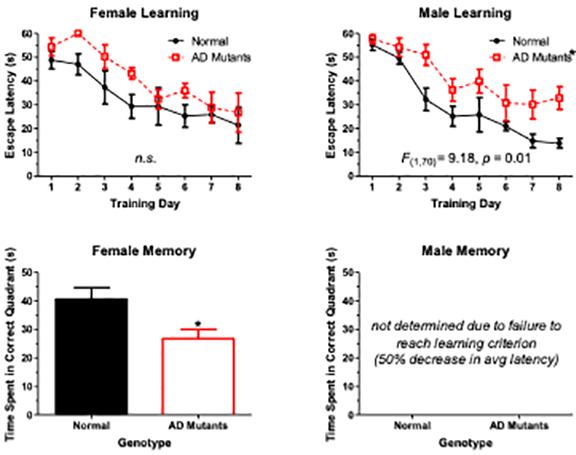
Notes:
Tg2576 animals exhibit changes in locomotor activity when compared to the wild types. This is measured in the open field arena. Video analysis of the behavior allows for heat maps to show where the animals have spent time. The time spent in the center or periphery of the box can analyzed further, and the graphs show that the mutants move more than the wildtypes, and they tend spend more time in the center of the box, a rough measure of anxiety. However, there are no genotype differences when you put these animals on the elevated plus maze, a better measure of anxiety. Since locomotor activity is related to dopamine levels in the brain, it can therefore be suggested that dopamine neurochemistry is altered in the AD mutants, but such changes are not related to differences in anxiety-related behaviors.
Tg2576/APPSwe mouse mutants have cognitive deficits that can be detected with the Morris watermaze. Tracking of wildtypes show that they will find a hidden escape platform (star) relatively quickly, but AD mice will wander around and might never find the platform. When these data are graphed you can see that female AD mice learn as well as the wildtypes, but AD males never learn the task (less than a 50% reduction in escape latency). However, when the escape platform is removed, the AD females do not remember the maze quadrant to which they were trained as well as the wildtypes. AD Males never learned the task, so running the memory probe on them is pointless.
Dietary treatment approaches available; animal costs dependent on their age at delivery and length of treatment desired
Optional Endpoints:
- Contextual Fear Conditioning (≥6 months)
- Hippocampal LTP/LTD (≥16 months)
- Oxidative stress enzymology (e.g. superoxide dismutase, catalase, glutathione, lipid peroxidation)
- Blood and tissue collections
- Brain area dissections
- Cytokine/chemokine analysis of blood and brain via Luminex(R)
- Other sandwich ELISAs
- CBC/clinical chemistry analysis
- Histopathologic analysis
- Immunohistochemistry analysis
Sample Data: BBP AD Model Overview
We can also support your In Vivo work with In Vitro studies: BBP In Vitro AD models
References
- Vossel, K.A., et al., Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol, 2017. 16(4): p. 311-322.
- Brzecka, A., et al., Sleep Disorders Associated With Alzheimer’s Disease: A Perspective. Front Neurosci, 2018. 12: p. 330.
- Alzheimer’s disease Facts and Figures. Alzheimer’s Associations, 2019.
- https://www.alzforum.org/research-models/tg2576
Related Pages
- Glioblastoma Models in Mice
- Epilepsy Models in Rats and Mice
- Addiction Models in Rats and Mice
- Cognition Models in Rats and Mice
- Experimental Autoimmune Encephalomyelitis (EAE)
- Developmental Milestones Aka Functional Observation Battery
- Anxiety Models in Rats and Mice
- Depression Model in Rat
- Fragile X Syndrome (FXS) in Mice
- Parkinson’s Disease Models in Rats and Mice
- Alzheimer’s Disease Model in Mice

