Experimental Autoimmune Encephalomyelitis (EAE)

Intro:
Experimental autoimmune encephalomyelitis (EAE) is a demyelinating central nervous system (CNS) disease that is commonly used as a model of Multiple Sclerosis (MS) since they share clinical and pathological features1-5,8-9,14,16-17. While the exact etiology of MS is unknown, rodent models have allowed for the better understanding of the inflammatory process over the course of the disease, from which many of the drugs that are currently in use in MS, such as glatiramer aceteate and natalizumab, have been developed or tested in EAE studies1-2, 6-10, 12, 15. This model allows for the development and preclinical testing of a wide range of potential therapeutic interventions as well as insight into immunopathogenic mechanisms of MS2,4,6-10,14.
Induction & Disease Parameters:
On day 0 of the study, mice will be anesthetized with 3% isoflurane and immunized with 200mg of MOG(35-55) in 100μL of complete Freund’s adjuvant (CFA) containing 4mg/ml of inactivated M. tuberculosis, in two subcutaneous injection sites on the back. One to two hours post the subcutaneous injections, the mice will receive 100μL of 500ng pertussis toxin (PTX) IP. A second injection of PTX will be administered IP 24 hours after the first injection.
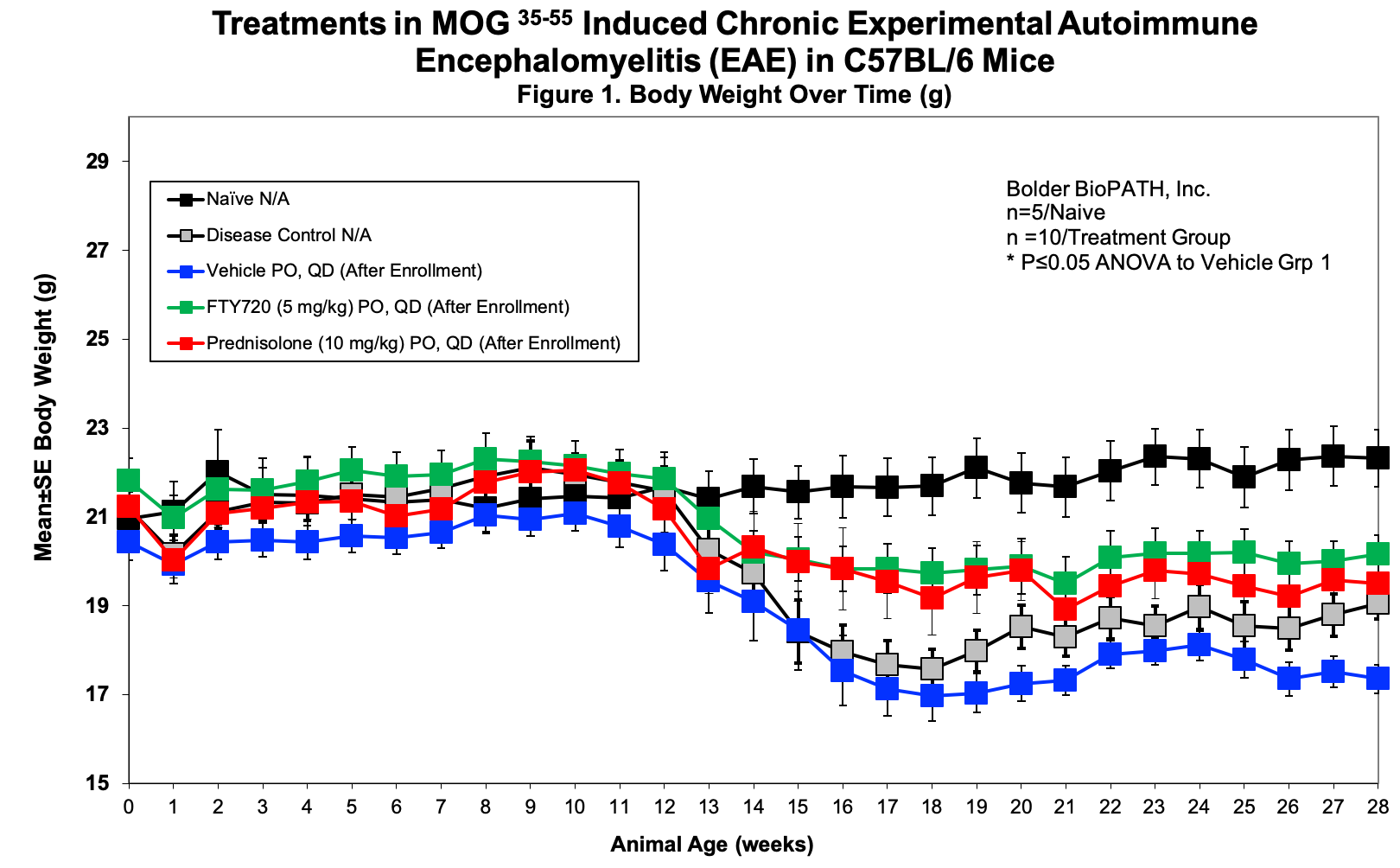
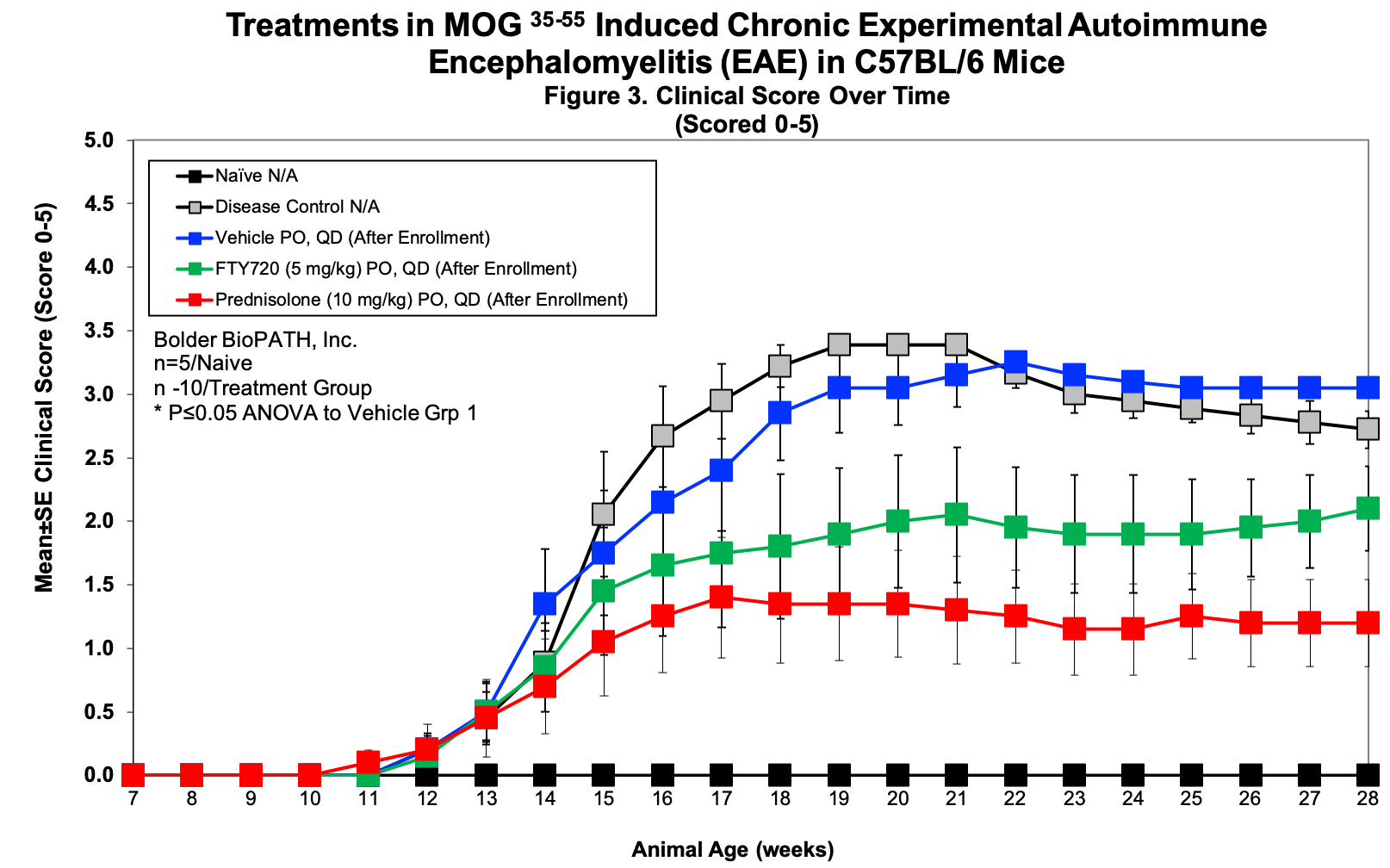
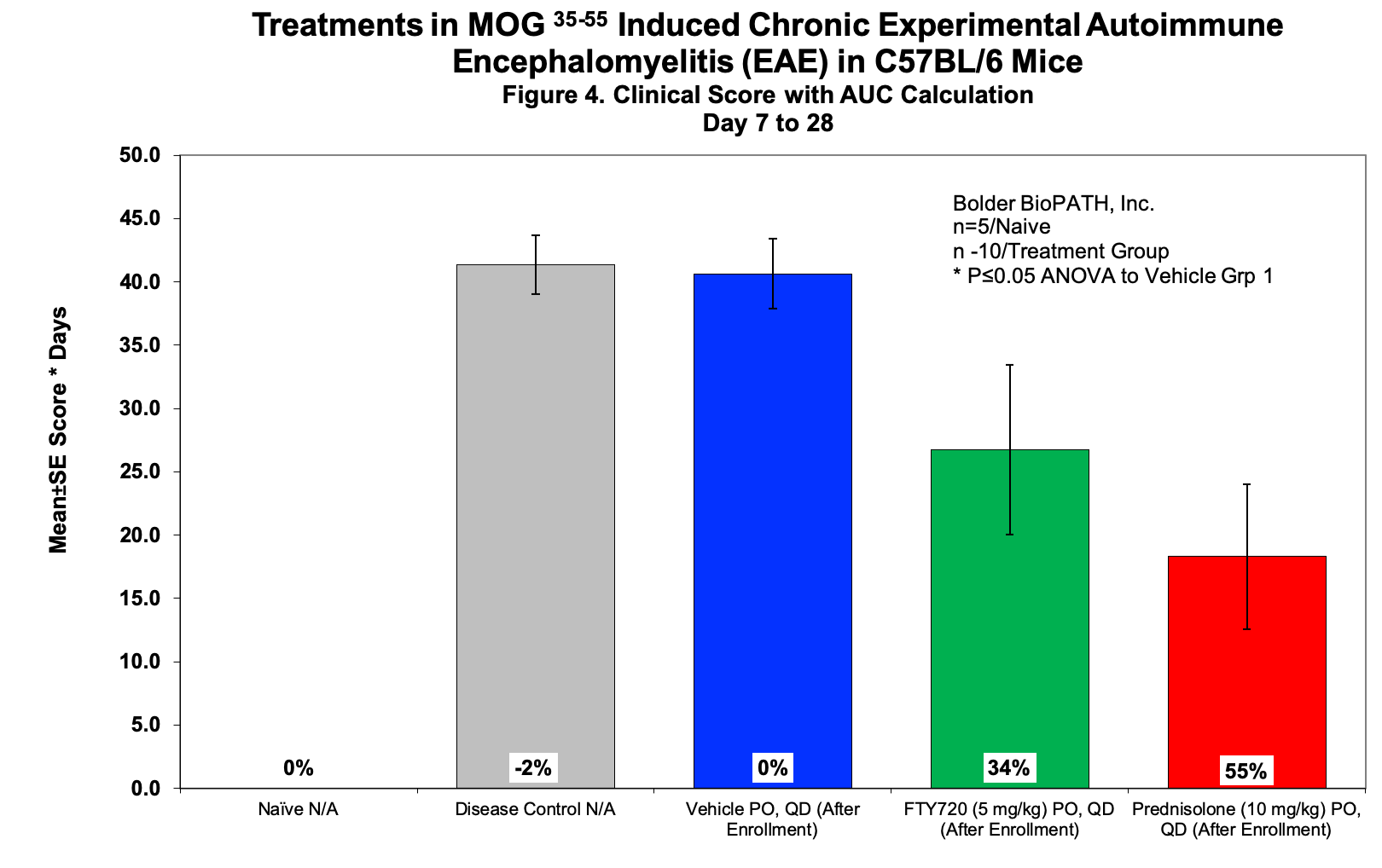
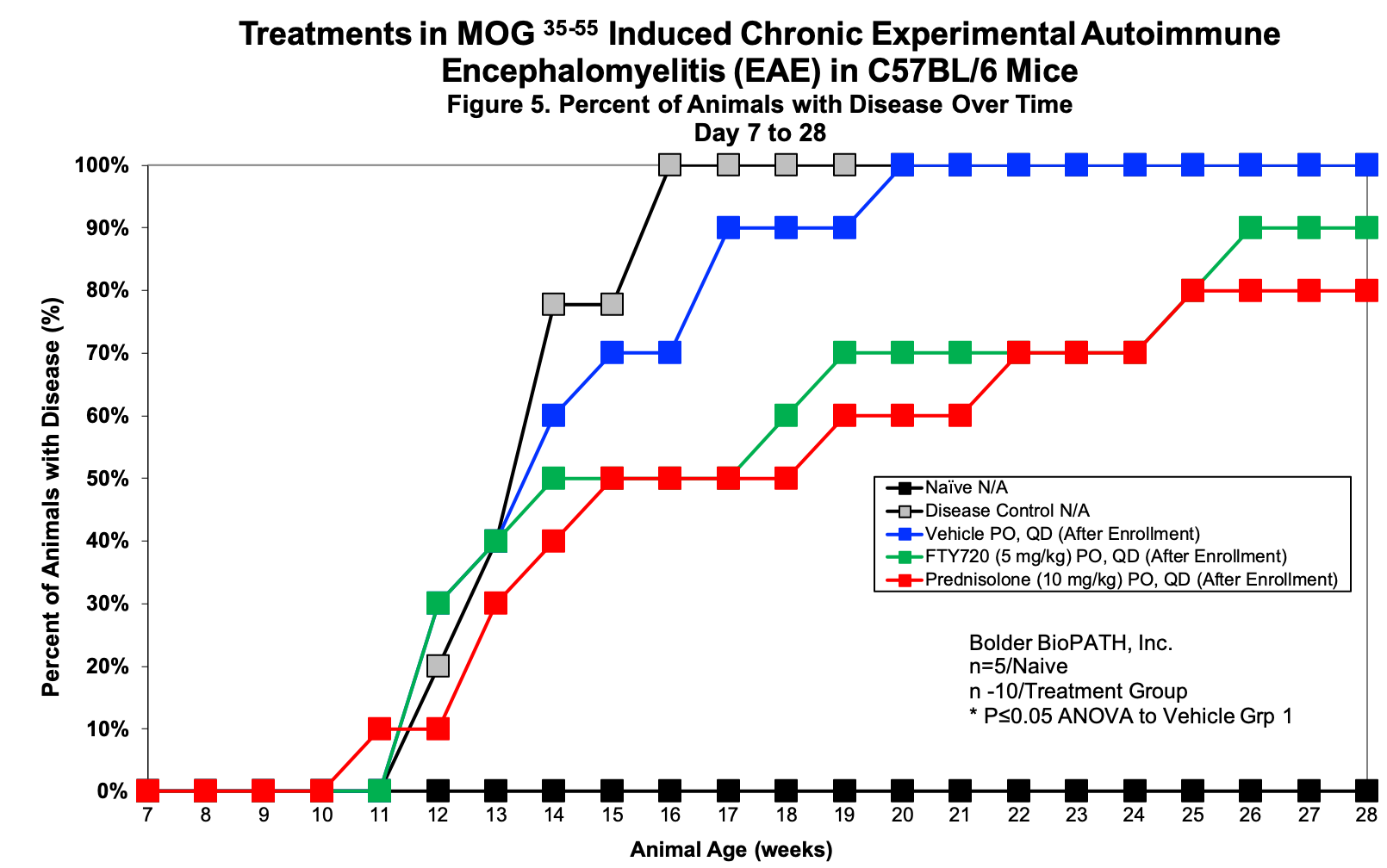
Animals will be clinical scored (see below) daily or every other day beginning on day 7 and weighed a minimum of 3 times per week for the duration of the study. Typically, 90-100% of mice begin showing signs of ataxia and paralysis by day 9-14 with maximum severity (full hind limb paralysis) occurring approximately three weeks after immunization, after which time clinical symptoms recede without returning to baseline. Studies will last no longer than 28 days.
Dosing Paradigms:
- Developing (Prophylactic) – Begin dosing on study day 0 and continue until necropsy on day 28.
- Semi-Established (Prophylactic) – Begin dosing on study day 1-6 and continue until necropsy on day 28.
- Established (Therapeutic) – Begin dosing on study day 7 (or later) and continue until necropsy on day 28.
Clinical Assessment:
| Score | Clinical Observations |
|---|---|
| 0 | No obvious changes in motor function compared to non-immunized mice. When picked up by base of tail, the tail has tension and is erect. Hind legs are usually spread apart. When the mouse is walking, there is no gait or head tilting. |
| 0.5 | Tip of tail is limp. When picked up by base of tail, the tail has tension except for the tip. Muscle straining is felt in the tail, while the tail continues to move. |
| 1 | Limp tail. When picked up by base of tail, instead of being erect, the whole tail drapes over finger. Hind legs are usually spread apart. No signs of tail movement are observed. |
| 1.5 | Limp tail and hind leg inhibition. When picked up by base of tail, the whole tail drapes over finger. When the mouse is dropped on a wire rack, at least one hind leg falls through consistently. Walking is very slightly wobbly. |
| 2 | Limp tail and weakness of hind legs. When picked up by base of tail, the legs are not spread apart, but held closer together. When the mouse is observed walking, it has a clearly apparent wobbly walk. One foot may have toes dragging, but the other leg has no apparent inhibitions of movement. – OR – Mouse appears to be at score 0.0, but there are obvious signs of head tilting when the walk is observed. The balance is poor. |
| 2.5 | Limp tail and dragging of hind legs. Both hind legs have some movement, but both are dragging at the feet (mouse trips on hind feet). – OR – No movement in one leg/completely dragging one leg, but movement in the other leg. – OR – EAE severity appears mild when picked up (as score 0.0-1.5), but there is a strong head tilt that causes the mouse to occasionally fall over. |
| 3 | Limp tail and complete paralysis of hind legs (most common). – OR – Limp tail and almost complete paralysis of hind legs. One or both hind legs are able to paddle, but neither hind leg is able to move forward of the hind hip. – OR – Limp tail with paralysis of one front and one hind leg. – OR – ALL of: Severe head tilting, walking only along the edges of the cage, Pushing against the cage wall, spinning when picked up by base of tail. |
| 3.5 | Limp tail and complete paralysis of hind legs. In addition to: Mouse is moving around the cage, but when placed on its side, is unable to right itself. Hind legs are together on one side of body. – OR – Mouse is moving around the cage, but the hindquarters are flat like a pancake, giving the appearance of a hump in the front quarters of the mouse. |
| 4 | Limp tail, complete hind leg and partial front leg paralysis. Mouse is minimally moving around the cage but appears alert and feeding. Often euthanasia is recommended after the mouse scores 4.0 for 2 days. However, with daily SC fluids some mice can recover to 3.5 or 3.0. When the mouse is euthanized because of severe paralysis, a score of 5.0 is entered for that mouse for the rest of the experiment. |
| 4.5 | Complete hind and partial front leg paralysis, no movement around the cage. Mouse is not alert. Mouse has minimal movement in the front legs. The mouse barely responds to contact. Euthanasia is recommended. When the mouse is euthanized because of severe paralysis, a score of 5.0 is entered for that mouse for the rest of the experiment. |
| 5 | Mouse is spontaneously rolling in the cage (euthanasia is recommended). – OR – Mouse is found dead due to paralysis. – OR – Mouse is euthanized due to severe paralysis. |
Histopathological Assessment:
Spinal cords from all surviving animals are examined microscopically by a board-certified veterinary pathologist.
Serial cervical, thoracic & lumbar sections will be stained by H&E for inflammatory infiltrate and luxol fast blue for demyelination. Foci of inflammatory cell infiltrate (sufficient size to be clearly recognizable as discreet foci-generally at least 50 µm in width) were counted and any small diffuse foci were assigned a value of 0.5. Any areas greater than 100 µm in width/length were divided and counted as multiple areas (example-a 100 X 500 µm lesion = 5 foci). Linear foci in meninges (usually 10-40 µm thick/various lengths) were counted in increments of 200 µm. These numbers were used to determine and inflammation score using the following criteria. Scores (based on counts) may be adjusted to reflect the severity of the disease present in controls.
Spinal Cord/Meninges
0=normal, none
0.5=very minimal, 1 or minimal diffuse (no distinct aggregates) in an area
1=minimal, 2-4 small discreet foci generally less than 100 µm width/length, may have
some minimal diffuse areas
2=mild, 5-7 small discreet foci, may have some minimal diffuse areas
3=moderate, 8-10 small plus larger coalescing areas
4=marked, 11-13 small plus larger coalescing areas
5=severe, often multiple coalescing >13
Brian/Meninges (Brain Stem Anterior, Brian Stem Posterior, Combined Cerebrum/Cerebellum)
0=normal, none
0.5=very minimal, 1-3
1=minimal, 4-10
2=mild, 11-17
3=moderate, 18-24
4=marked, 25-31
5=severe. >31
Percent area of white matter that had demyelination, edema, dilated axons was estimated and used to determine a score using the following criteria:
0=normal
0.5=very minimal, few scattered affected axons
1=minimal, 1-5% of total area affected
2=mild, 5-25% of total area affected
3=moderate, 26-50% of total area affected
4=marked, 51-75% of total area affected
5=>75% of total area affected
Sample Data:
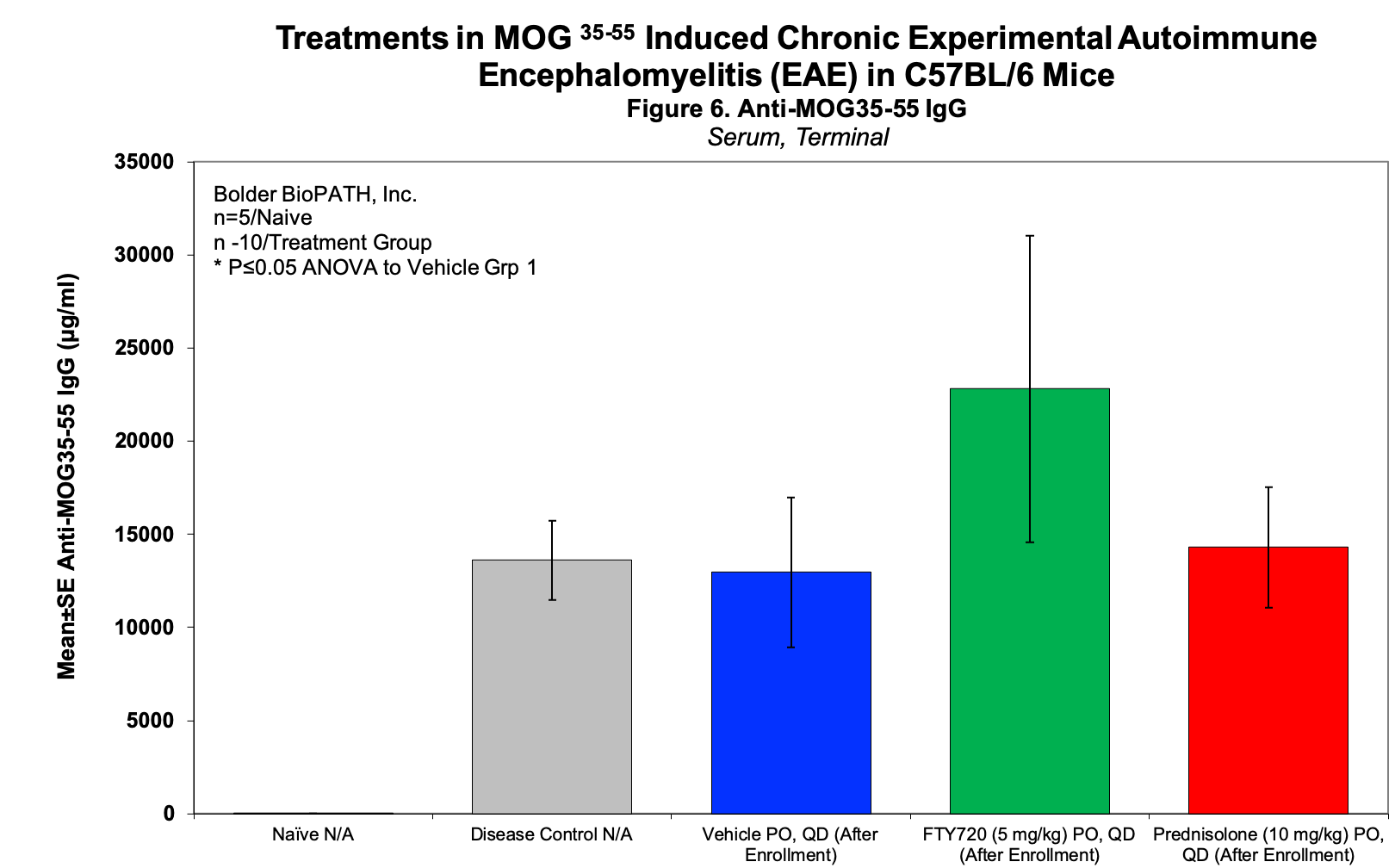
Endpoints:
– Clinical Score
– Body weight
– Histopathology
References:
- Friese MA, et al. The value of animal models for drug development in multiple sclerosis. Brain. 2006; 129: 1940-1952.
- Fletcher JM, et al. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clinical and Experimental Immunology. 2010; 162: 1-11.
- Stromnes IM, et al. Differential regulation of central nervous system autoimmunity by TH1 and TH17 cells. Nat Med. 2008; 14(3): 337-349.
- Cravens PD, et al. Lymph node-derived donor encephalitogenic CD4+ T cells in C57BL/6 mice adoptive transfer experimental autoimmune encephalomyelitis highly express GM-CSF and T-bet. Journal of Neuroinflammation. 2011; 8:73.
- Wolf SD, et al. Experimental autoimmune encephalomyelitis induction in genetically B cell deficient mice. J Exp Med. 1996; 184: 2271-2278.
- Tafreshi AP, Mostafavi H, and Zeynali B. Induction of experimental allergic encephalomyelitis in C57/BL6 mice: an animal model for multiple sclerosis. Iranian J of Allergy, Asthma, and Immunology. 2005; 4(3).
- Mangas A, et al. Evaluation of the effects of a new drug candidate (GEMSP) in a chronic EAE model. Int J Biol Sci. 2008; 4.
- Bittner S, et al. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J of Vis Exp. 2014; 86.
- Constantinescu CS, et al. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). British J of Pharmacology. 2011; 164: 1079-1106.
- Donia M, et al. Specific and strain-independent effects of dexamethasone in the prevention and treatment of experimental autoimmune encephalomyelitis in rodents. Scandinavian J of Immunology. 2010; 72: 396-407.
- Fan YP, et al. Protective effect of tanreqing injection on axon myelin damage in the brain of mouse model for experimental autoimmune encephalomyelitis. J Tradit Chin Med. 2014; 34(5): 576-583.
- Green LK, et al. Enhanced disease reduction using clozapine, an atypical antipsychotic agent, and glatiramer acetate combination therapy in experimental autoimmune encephalomyelitis. Multiple Sclerosis Journal—Experimental, Translational and Clinical. 2017; 1-13.
- Khan N and Smith MT. Multiple sclerosis-induced neuropathic pain: pharmacological management and pathophysiological insights from rodent EAE models. Inflammopharmacol. 2014; 22: 1-22.
- Lyons JA, et al. B cells are critical to induction of experimental allergic encephalomyelititis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999; 29: 3432-3439.
- Rangachari M and Kuchroo VK. Using EAE to better understand principles of immune function and autoimmune pathology. J Autoimmun. 2013; 45: 31-39.
- Thakker P, et al. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J of Immunol. 2007; 178(4): 2589-2598.
- Thakker P, et al. Cytosolic phospholipase A2a blockade abrogates disease during the tissue-damage effector phase of experimental autoimmune encephalomyelitis by its action on APCs. J Immunol. 2011; 187: 1986-1997.
Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013; 19(2):15-20.
Related Pages
- Glioblastoma Models in Mice
- Epilepsy Models in Rats and Mice
- Addiction Models in Rats and Mice
- Cognition Models in Rats and Mice
- Experimental Autoimmune Encephalomyelitis (EAE)
- Developmental Milestones Aka Functional Observation Battery
- Anxiety Models in Rats and Mice
- Depression Model in Rat
- Fragile X Syndrome (FXS) in Mice
- Parkinson’s Disease Models in Rats and Mice
- Alzheimer’s Disease Model in Mice